Clinical research in the palm of your hand!
The mobility and versatility of TrialKit allows for seamless regulatory compliant (21 CFR Part 11) data capture from mobile devices anytime, anywhere. Once collected, data can be easily aggregated, analyzed and shared, making collaboration between research teams more productive.
TrialKit is perfect for CROs, Sponsors, Academic Institutions, and Individual Researchers.
Build a study
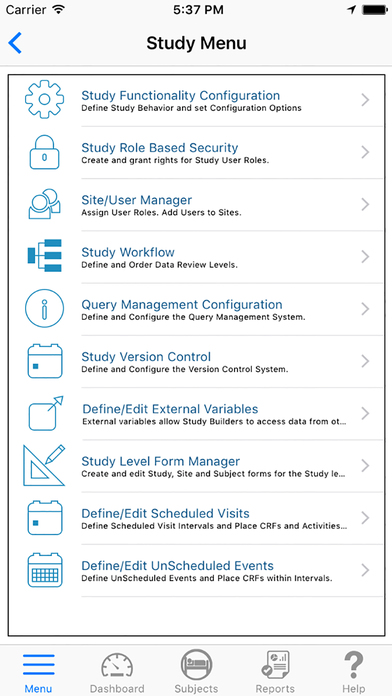
An intuitive user interface enables study builders to create electronic case report forms (eCRFs) to collect essential study data in accordance with study protocol and regulatory requirements. TrialKit lays the foundation for a truly paperless and compliant study. With no programming expertise, any clinical professional can create a study using any iOS device.
Manage a study
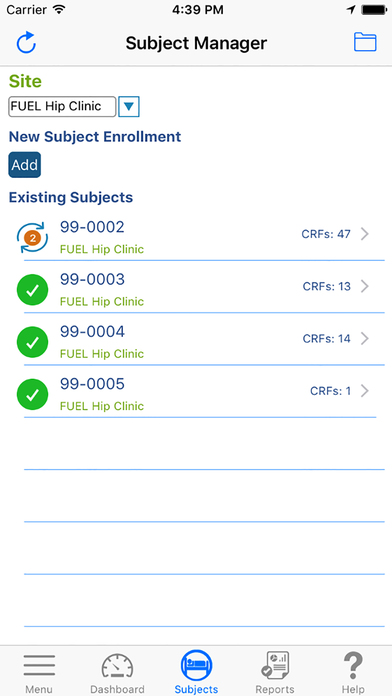
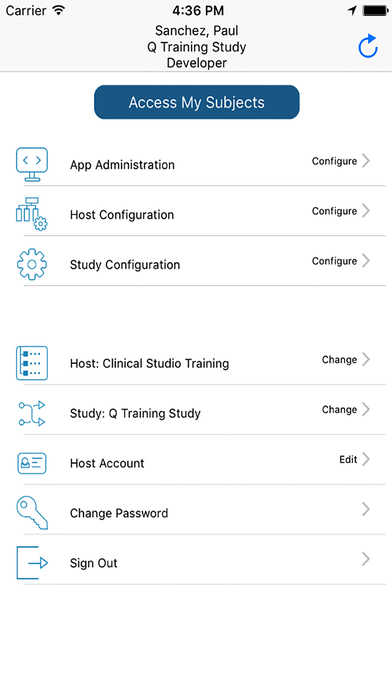
With TrialKit, clinical research can be expertly managed from start to finish on an iOS device. Research professionals are truly capable of managing a clinical trial from anywhere, whether they are on or off site. The ability to access, monitor, and review data or respond to queries on-the-go is now a reality.
Maximize productivity, minimize cost
Every clinical trial is different, so prices are tailored to fit each one. Regardless of the study size, TrialKits objective is to drastically reduce the cost of clinical research and make it more available. Download now and you get full access to the system for 60 days, free of charge.
Feature and Functionality:
Study Manager
Create, edit, and archive studies
Define/Edit Randomization
Define randomization events, stratification, and blinding
Regulatory Audit Reports
Report on all changes throughout the system
User Manager
Add or edit users to host. View user information. Add content to user forms
Define/Edit Scheduled Visits
Define scheduled visit intervals and place CRFs and activities within intervals
Clinical/Medical Coding
WHO Drug (B3/C3) certified. MedDRA certified
Study Workflow
Define and order data review levels
Form Manager
Design and edit study, site and subject forms
Query Management Configuration
Define and configure the query management system
Uses the Power of iOS
Program Push Notifications, Stamp GPS location to any record and view on map, Operates in both Landscape and Portrait modes on any iOS device from an iPhone 5 on up, Take pictures or videos directly into CRFs, Sign case report forms with your finger or stylus
MUCH, MUCH More!